Portable water: It's not at all suited to general pharmaceutical use due to the considerable quantity of dissolved solids (chlorides, sulphates and bicarbonates of Na, K, Ca and Mg existing.
Bradley: "And so we must start to suitable this issue, we definitely should comprehend it. So it is nice this details is coming out, and to try this We've got to get launch of latest details."
These outcomes are in the light of the latest results in our lab, during which 3T3 fibroblasts and HaCaT keratocytes derived from SHS-induced 3D spheroids revealed significant morphological improvements [31].
Ordinarily, few issues are encountered in maintaining the chemical purity of Purified Water and Water for Injection However, the advent of making use of conductivity and TOC to outline chemical purity has allowed the consumer to additional quantitatively evaluate the water's chemical purity and its variability like a perform of regimen pretreatment system upkeep and regeneration. Even the existence of this sort of unit functions as warmth exchangers and use point hoses can compromise the chemical good quality of water within and shipped from an usually perfectly-managed water program. Thus, an assessment on the regularity with the water's chemical purity with time need to be A part of the validation software. Even so, even with one of the most well controlled chemical high quality, it is frequently more challenging to persistently satisfy proven microbiological high quality standards owing to phenomena occurring throughout and after chemical purification. A typical system will involve intensive day-to-day sampling and screening of key procedure points for a minimum of just one month right after operational requirements are recognized for each device Procedure, level of use, and sampling issue.
Reverse osmosis can be a membrane-centered procedure which removes substances dissolved from the water and is particularly used to desalinate the feedwater. The reverse osmosis operates on the next theory:
This water is packaged and rendered sterile. It is actually used for planning of sterile products and solutions or in analytical programs requiring purified water when usage of a validated system is not really functional and only a little quantity is needed. It is additionally used when bulk packaged purified water just isn't suitably microbiologically managed.
An archaic comprehension of microbial retentive filtration would guide a single to equate a filter's score Using the Phony effect of a simple sieve or display that Certainly retains particles sized at or above the filter's score.
The development of RO units which will tolerate sanitizing water temperatures as well as function proficiently and consistently at elevated temperatures has added tremendously to their microbial website Regulate and to the avoidance of biofouling.
The Ultra Filtration process will accomplish an automated backwash just after every single settable time of operation cycle or if differential pressure exceeds greater than 1 bar. Backwash frequency of UF is settable on HMI and subject to alter dependant on incoming load of suspended solids at UF.
Microbial-Retentive Filtration Microbial-retentive membrane filters have expert an evolution of comprehension previously 10 years which has caused Beforehand held theoretical retention mechanisms to be reconsidered. These filters have a larger successful “pore size” get more info than ultrafilters and therefore are intended to reduce the passage of microorganisms and equally sized particles without unduly proscribing move. This type of filtration is extensively used within just water devices for filtering the micro organism away from equally water and compressed gases and for vent filters on tanks and stills along with other unit operations. Nevertheless, the properties on the water technique microorganisms seem to obstacle a filter's microbial retention from water with phenomena absent from other aseptic filtration applications, including filter sterilizing of pharmaceutical formulations just before packaging. During the latter application, sterilizing quality filters are frequently viewed as to own an assigned rating of 0.
The really hydrophobic Get hold of angle observed for the culture liquid-coating interface is an efficient situation with the 3D spheroid improvement through incubation of Uncooked 264.seven murine macrophages (forty eight h). The impact of two Preliminary mobile densities (two hundred and 2000 cel/μL) within the formation with the spheroids was investigated. The outcome of substrate (agarose or SHS) demonstrated considerable distinctions between the circularity values for the aggregates generated at the best cell density (2000 cel/μL). In the situation of dimension distribution, important differences had been located in all circumstances. These results instructed that SHS shown improved attributes to the 3D aggregates to be a functionality with the imposed compositions because of the development of denser, smaller sized aggregates as compared to People shaped on agarose hydrogel. The geometrical properties (circularity and measurement distribution) in the RAW264.7-organized spheroids are comparable with our earlier effects on SHS-induced 3D aggregates [30,31]. In such cases, the noticed decreased density and compactness could possibly be affiliated with the included mobile line.
Glance very carefully for just about any cross-connections on the potable water supply. Non-potable water provide traces ought to be Obviously marked as a result, particularly when adjacent to potable water offer connections.
The smoothness and composition from the floor might influence the speed of Preliminary microbial adsorption, but once adsorbed, biofilm growth, unless in any other case inhibited by sanitizing disorders, will occur whatever the surface area. The moment fashioned, the biofilm turns into a continual supply of microbial contamination.
Thing to consider should also be specified into the timeliness of microbial enumeration screening just after sample collection. The amount of detectable planktonic micro organism within a sample collected within a scrupulously clean up sample container will often drop as time passes. The planktonic microbes within the sample will are likely to possibly die or to irretrievably adsorb to the container walls lessening the number of viable planktonic microorganisms which might be withdrawn in the sample for tests.
 Luke Perry Then & Now!
Luke Perry Then & Now!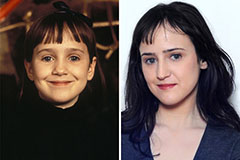 Mara Wilson Then & Now!
Mara Wilson Then & Now! Richard "Little Hercules" Sandrak Then & Now!
Richard "Little Hercules" Sandrak Then & Now! Elisabeth Shue Then & Now!
Elisabeth Shue Then & Now! Heather Locklear Then & Now!
Heather Locklear Then & Now!